Publications
Publications | Database | Reactivity Scales Poster
Publications
| 134.* | Introducing TeHyperBTM as an Organocatalyst for (4+2)-Cycloadditions of Michael Acceptors with Allenoates, But-2-ynoates or Allenylphosphonates M. Piringer, A. Scheucher, M. Hofer, M. Bechmann, A. R. Ofial,* L. Stockhammer,* M. Waser,* ChemistryEurope 2026, 4, e202500443. Open Access:  |
| 133.* | Quantifying the enamine-type nucleophilic reactivity of α-aryl vinyl azides P. Thiruvengetam, J. Brossette, C. Gross, H. Zipse,* A. R. Ofial,* Chem. Commun. 2026, Advance Article (DOI: 10.1039/d6cc00190d). Open Access:  FAIR data at Open Data LMU (DOI: 10.5282/ubm/data.761) |
| 132.* | Quantifying the Electrophilicity of 9(10H)-Phenanthrenone- and 1-Acenaphthenone-Derived α,β-Unsaturated Ketones C. Gross, J. Brossette, L. Schellmann, P. Mayer, H. Zipse,* A. R. Ofial,* J. Phys. Org. Chem. 2026, 39, e70061. Open Access:  FAIR kinetics data at Open Data LMU (DOI: 10.5282/ubm/data.677) |
| 131. | Stepwise or concerted? One-bond nucleophilicity and -electrophilicity parameters for the mechanistic analysis of 1,3-dipolar cycloadditions H. Mayr, L. Li, R. J. Mayer, A. R. Ofial, Pure Appl. Chem. 2026, 98, 97-113. Access:  |
| 130. | Basicities and Nucleophilicities of N-Centered Organocatalysts H. Mayr, A. R. Ofial In Enantioselective Organocatalysis: Catalysts, Reactions and Applications (P. Dalko, ed.), Wiley-VCH, Weinheim, 2025, Chapter 9, pp. 319-354. Access:  |
| 129.* | From Oxygen to Tellurium: The Impact of the Chalcogen on Nucleophilicities and Basicities of Isochalcogenourea Catalysts Von Sauerstoff zu Tellur: Der Einfluss des Chalkogenatoms auf die Nucleophilie und Basizität von Isochalkogenharnstoff-basierten Katalysatoren L. Stockhammer, K. Kasten, A. Eitzinger, L. S. Vogl, M. Piringer, D. Weinzierl, A. R. Ofial,* A. D. Smith,* M. Waser,* Angew. Chem. Int. Ed. 2025, 64, e202514865. Open Access:  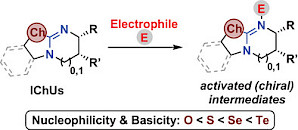 |
| 128. | How Electrophilic Are Keteniminium Ions? T. Siegmund, D. S. Stephenson, A. R. Ofial, R. J. Mayer, H. Mayr, Synthesis 2025, 57, 3251–3262. Access:  |
| 127. | Revisiting Mayr’s reactivity database: expansion, sensitivity analysis, and uncertainty quantification M. K.-E. Wolff, A. R. Ofial, J. Proppe, Org. Biomol. Chem. 2025, 23, 7188-7196. Open Access:  |
| 126.* | Reactivity of δ-Functionalized para-Quinone Methides in Nucleophilic Addition Reactions C. Gross, A. Eitzinger, P. Mayer, A. R. Ofial,* Chem. Eur. J. 2025, 31, e202501224. Open Access:  FAIR kinetics data at Open Data LMU (DOI: 10.5282/ubm/data.582) 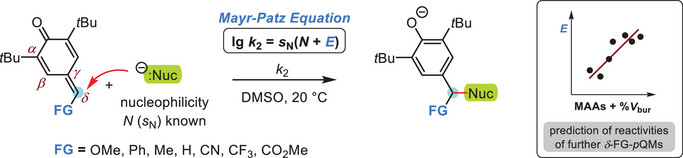 |
| 125.* | Pyridinamide Ion Pairs: Design Principles for Super-Nucleophiles in Apolar Organic Solvents V. Burger, M. Franta, A. C. O‘Donoghue,* A. R. Ofial,* R. M. Gschwind,* H. Zipse,* J. Org. Chem. 2025, 90, 2298-2306. Open Access:  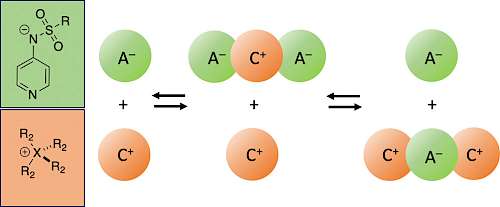 |
| 124.* | Highly Nucleophilic Pyridinamide Anions in Apolar Organic Solvents due to Asymmetric Ion Pair Association V. Burger, M. Franta, A. R. Ofial,* R. M. Gschwind,* H. Zipse,* J. Am. Chem. Soc. 2025, 147, 5043-5050. Open Access:  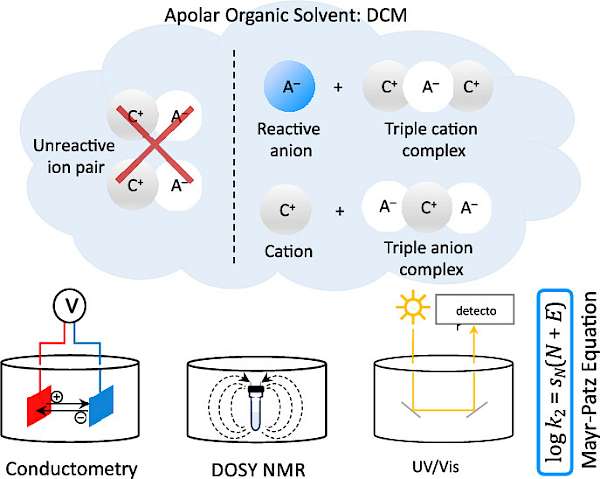 |
| 123. | Asymmetric isochalcogenourea-catalysed (4+2)-cycloadditions of ortho-quinone methides and allenoates A. Scheucher, C. Gross, M. Piringer, J. Novacek, A. R. Ofial, M. Waser, Org. Biomol. Chem. 2025, 23, 827-834. Open Access:  FAIR kinetics data at Open Data LMU (DOI: 10.5282/ubm/data.545) 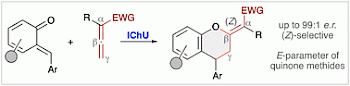 |
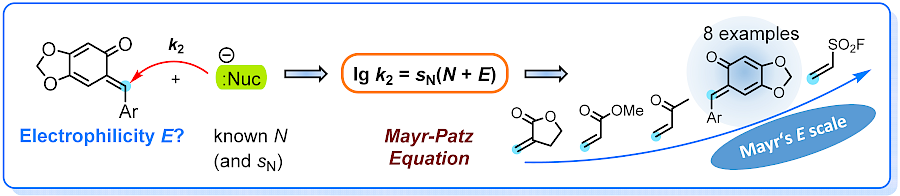
| 122.* |
Defining the Synthetic Scope of ortho-Quinone Methides by Quantifying Their Electrophilicity |
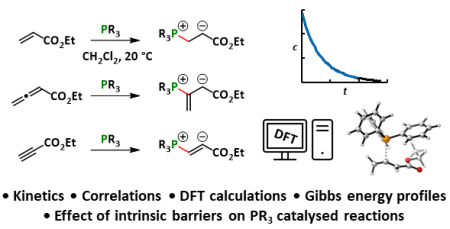
| 121.* |
Reactivities of tertiary phosphines towards allenic, acetylenic, and vinylic Michael acceptors |
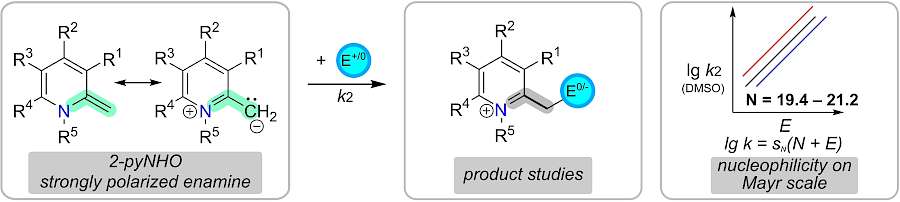
| 120.* |
2-Methylene-1,2-dihydropyridines (2-pyNHOs): Highly Nucleophilic Enamines |
| 119.* | Lactone Enolates of Isochroman-3-ones and 2-Coumaranones: Quantification of Their Nucleophilicity in DMSO and Conjugate Additions to Chalcones M. S. Mousavi, A. Di Mola, G. Pierri, C. Tedesco, M. J. Hensinger, A. Sun, Y. Wang, P. Mayer, A. R. Ofial,* A. Massa,* J. Org. Chem. 2024, 89, 6915-6928. Open Access:  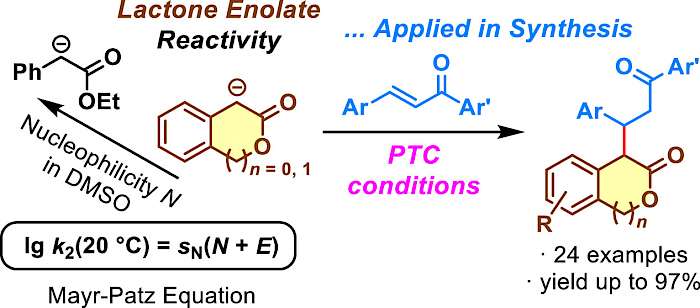 |
| 118.* | Reactivity of Electrophilic Trifluoromethylating Reagents D. S. Timofeeva, Á. Puente, A. R. Ofial,* H. Mayr,* Eur. J. Org. Chem. 2024, 27, e202400085. Open Access:  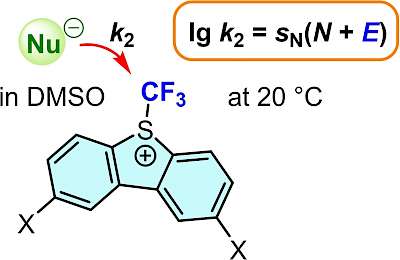 |
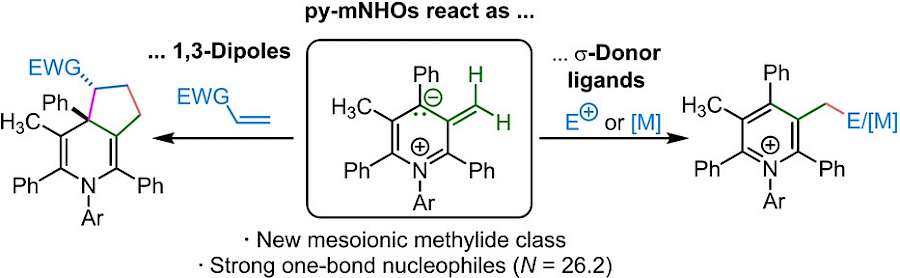
| 117.* |
Pyridinium-Derived Mesoionic N-Heterocyclic Olefins (py-mNHOs) |
| 116.* | Unveiling the impact of a CF2 motif in the isothiourea catalyst skeleton: Evaluating C(3)-F2-HBTM and its catalytic activity M. T. Westwood, K. Kasten, L. Stockhammer, R. del Río-Rodríguez, J. A. Fernández-Salas, A. Eitzinger, A. M. Z. Slawin, M. Waser, J. Alémán, A. R. Ofial,* A. D. Smith,* ARKIVOC 2024 (4) 202312093. Open Access:  |
| 115.* | Nucleophilicity of 4-(Alkylthio)-3-imidazoline Derived Enamines M. J. Hensinger, A. Eitzinger, O. Trapp,* A. R. Ofial,* Chem. Eur. J. 2024, 30, e202302764. Selected as "Hot Paper"! Open Access:  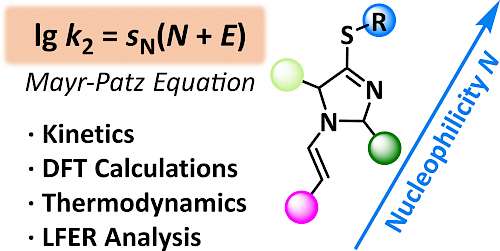 |
| 114.* |
Pushing the Upper Limit of Nucleophilicity Scales by Mesoionic N-Heterocyclic Olefins |
| 113.* | The effect of S-alkylation on organocatalytic enamine activation through imidazolidine-4-thiones M. J. Hensinger, A. C. Closs, O. Trapp,* A. R. Ofial,* Chem. Commun. 2023, 59, 8091-8094. Open Access: 
|
| 112. | When Does Hammond's Postulate Predict Stabilities of Carbocations? H. Mayr, A. R. Ofial, Isr. J. Chem. 2023, 63, e202300054. Open Access:  |
| 111.* | Reactivity of Electrophilic Cyclopropanes A. Eitzinger, A. R. Ofial,* Pure Appl. Chem. 2023, 95, 389-400. Open Access:  FAIR kinetics and NMR data at Open Data LMU (DOI: 10.5282/ubm/data.685) |
| 110.* |
One-Bond-Nucleophilicity and -Electrophilicity Parameters: An Efficient Ordering System for 1,3-Dipolar Cycloadditions
|
| 109.* |
Nucleophilicities of Cyclic α-Diazo Carbonyl Compounds
|
| 108. | Elucidation of the Nucleophilic Potential of Diazocyclopentadiene M. Hartnagel, A. R. Ofial, H. Mayr, Synthesis 2023, 55, 354-358. Access: |
| 107. | Resolving the Mechanistic Complexity in Triarylborane-Induced Conjugate Additions R. J. Mayer, N. Hampel, A. R. Ofial, H. Mayr, ACS Catal. 2022, 12, 15298-15309. Access:  |
| 106.* | Cyclobutane Formation by the Reaction of Ethenesulfonyl Fluoride with Dimethyl Diazomalonate L. Li, P. Mayer, A. R. Ofial,* H. Mayr,* Eur. J. Org. Chem. 2022, e202200865. Open Access:  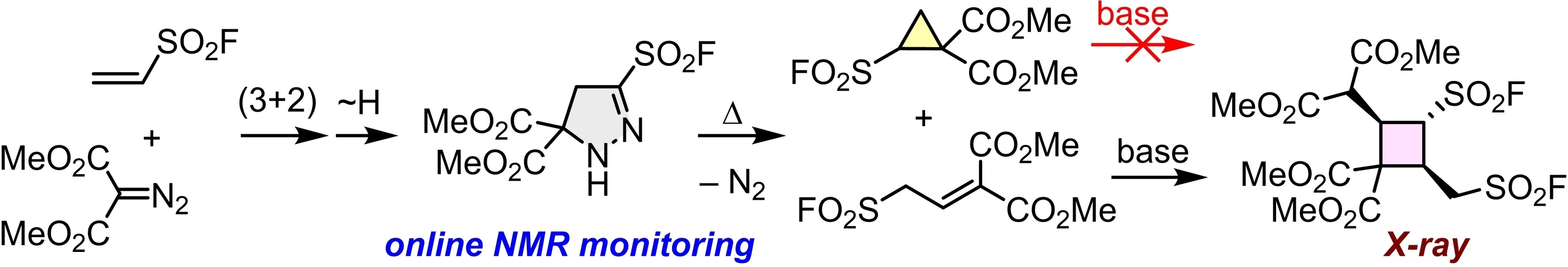 |
| 105.* | Quantification of the Electrophilicities of Diazoalkanes: Kinetics and Mechanism of Azo Couplings with Enamines and Sulfonium Ylides L. Li, R. J. Mayer, D. S. Stephenson, P. Mayer, A. R. Ofial,* H. Mayr,* Chem. Eur. J. 2022, 28, e202201376. Selected as "Hot Paper"! Open Access:  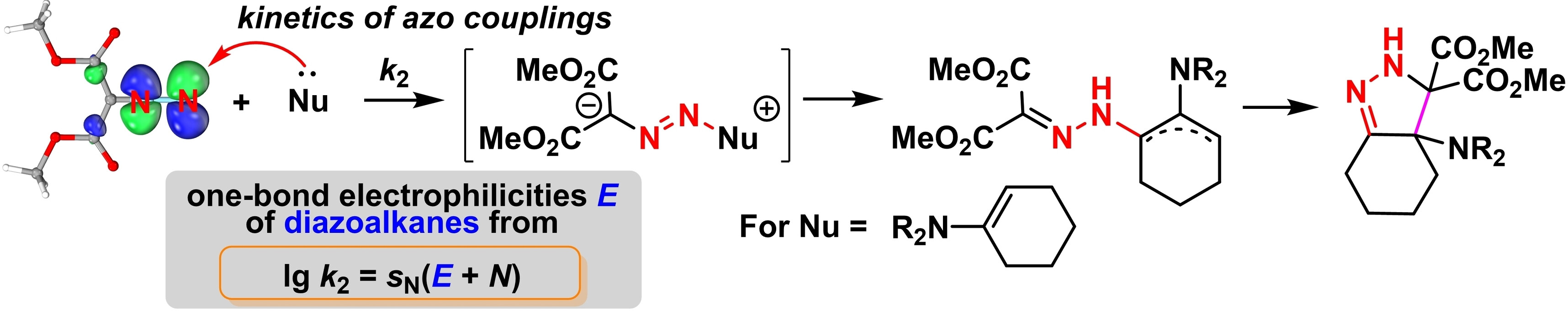 |
| 104. | Epigenetic anti-cancer treatment with a stabilized carbocyclic Decitabine analogue F. R. Traube, N. F. Brás, W. P. Roos, C. C. Sommermann, T. Diehl, R. J. Mayer, A. R. Ofial, M. Müller, H. Zipse, T. Carell, Chem. Eur. J. 2022, 28, e202200640. Open Access:  |
| 103. | Access to β-Alkylated γ-Functionalized Ketones via Conjugate Additions to Arylidieneisoxazol-5-ones and Mo(CO)6-Mediated Reductive Cascade Reactions A. Macchia, F. F. Summa, G. Monaco, A. Eitzinger, A. R. Ofial, A. Di Mola, A. Massa, ACS Omega 2022, 7, 8808-8818. Open Access:  |
| 102.* | Reactivities of allenic and olefinic Michael acceptors towards phosphines F. An, H. Jangra, Y. Wei, M. Shi,* H. Zipse,* A. R. Ofial,* Chem. Commun. 2022, 58, 3358-3361. Open Access:  |
| 101. | Ein übersehener Reaktionsweg bei 1,3-dipolaren Cycloadditionen von Diazoalkanen mit Enaminen An Overlooked Pathway in 1,3-Dipolar Cycloadditions of Diazoalkanes with Enamines L. Li, P. Mayer, D. S. Stephenson, A. R. Ofial, R. J. Mayer, H. Mayr, Angew. Chem. 2022, 134, e202117047; Angew. Chem. Int. Ed. 2022, 61, e202117047. Selected as "Hot Paper"! Open Access:  |
| 100.* | Inherent Reactivity of Spiro-Activated Electrophilic Cyclopropanes P. M. Jüstel, A. Stan, C. D. Pignot, A. R. Ofial,* Chem. Eur. J. 2021, 27, 15928-15935. Selected as "Hot Paper"! Open Access:  Cover Feature: Chem. Eur. J. 2021, 27, 15824.    |
| 99.* | Base-Promoted Cascade Reactions for the Synthesis of 3,3-Dialkylated Isoindolin-1-ones and 3-Methyleneisoindolin-1-ones A. Macchia, F. F. Summa, A. Di Mola, C. Tedesco, G. Pierri, A. R. Ofial,* G. Monaco,* A. Massa,* J. Org. Chem. 2021, 86, 15128-15138. Open Access:  |
| 98. | Quantification of the Lewis Basicities and Nucleophilicities of 1,3,5-Tris(dialkylamino)benzenes G. Micheletti, R. J. Mayer, S. Cino, C. Boga, A. Mazzanti, A. R. Ofial, H. Mayr, Eur. J. Org. Chem. 2021, 6347-6357. Selected as "VIP Paper"! Open Access:  |
| 97.* | Dynamics of the Dimethyl Sulfide Exchange of (1,3- Diphenylallyl)dimethylsulfonium Ions P. M. Jüstel, P. Rovó, H. Mayr, A. R. Ofial,* J. Phys. Org. Chem. 2022, 35, e4270. (accepted 18-July-2021) Open Access:  |
| 96. | Nucleophilicities and Nucleofugalities of Thio- and Selenoethers B. Maji, X.-H. Duan, P. M. Jüstel, P. A. Byrne, A. R. Ofial, H. Mayr, Chem. Eur. J. 2021, 27, 11367-11376. Selected as "Hot Paper"! Open Access:  |
| 95.* | Nucleophilic Reactivities of Thiophenolates P. M. Jüstel, C. D. Pignot, A. R. Ofial,* J. Org. Chem. 2021, 86, 5965-5972. Access:  |
| 94.* | Electrophilic Reactivities of Cyclic Enones and α,β-Unsaturated Lactones R. J. Mayer, P. W. A. Allihn, N. Hampel, P. Mayer, S. A. Sieber, A. R. Ofial,* Chem. Sci. 2021, 12, 4850-4865. Open Access:  |
| 93.* | Lewis Acidic Boranes, Lewis Bases, and Equilibrium Constants: A Reliable Scaffold for a Quantitative Lewis Acidity/Basicity Scale R. J. Mayer, N. Hampel, A. R. Ofial,* Chem. Eur. J. 2021, 27, 4070-4080. Open Access:  |
| 92.* | From Carbodiimides to Carbon Dioxide: Quantification of the Electrophilic Reactivities of Heteroallenes Z. Li, R. J. Mayer, A. R. Ofial,* H. Mayr,* J. Am. Chem. Soc. 2020, 142, 8383-8402. Access:  |
| 91. | Voraussage absoluter Geschwindigkeitskonstanten von Huisgen- Reaktionen ungesättigter Iminium-Ionen mit Diazoalkanen Predicting Absolute Rate Constants for Huisgen Reactions of Unsaturated Iminium Ions with Diazoalkanes J. Zhang, Q. Chen, R. J. Mayer, J.-D. Yang, A. R. Ofial, J.-P. Cheng, H. Mayr, Angew. Chem. 2020, 132, 12628-12634; Angew. Chem. Int. Ed. 2020, 59, 12527-12533. Open Access:  |
| 90. | CF3-Containing para-Quinone Methides for Organic Syntheses M. Winter, R. Schütz, A. Eitzinger, A. R. Ofial, M. Waser, Eur. J. Org. Chem. 2020, 3812-3817. Open Access:  |
| 89.* | Electrophilic Reactivities of Vinyl p-Quinone Methides A. Eitzinger, R. J. Mayer, N. Hampel, P. Mayer, M. Waser, A. R. Ofial,* Org. Lett. 2020, 22, 2182-2186. Open Access:  |
| 88. | Lewis Acidity Scale of Diaryliodonium Ions toward Oxygen, Nitrogen, and Halogen Lewis Bases R. J. Mayer, A. R. Ofial, H. Mayr, C. Y. Legault, J. Am. Chem. Soc. 2020, 142, 5221-5233. Access:  |
| 87.* | Basicities and Nucleophilicities of Pyrrolidines and Imidazolidinones Used as Organocatalysts F. An, B. Maji, E. Min, A. R. Ofial,* H. Mayr,* J. Am. Chem. Soc. 2020, 142, 1526-1547. Access:  Highlighted as "Synfact of the Month" (06/2020) |
| 86.* | Nucleophilie von Glutathion als Bindeglied zur Reaktivität von Michael-Akzeptoren Nucleophilicity of Glutathione: A Link to Michael Acceptor Reactivities R. J. Mayer, A. R. Ofial,* Angew. Chem. 2019, 131, 17868-17872; Angew. Chem. Int. Ed. 2019, 58, 17704-17708. Open Access:  |
| 85.* | Ambident Reactivity of Phenolate Anions Revisited: A Quantitative Approach to Phenolate Reactivities R. J. Mayer, M. Breugst, N. Hampel, A. R. Ofial,* H. Mayr,* J. Org. Chem. 2019, 84, 8837-8858. Access:  |
| 84.* | Metal Enolates - Enamines - Enol Ethers: How Do Enolate Equivalents Differ in Nucleophilic Reactivity? A. I. Leonov, D. S. Timofeeva, A. R. Ofial,* H. Mayr,* Synthesis 2019, 51, 1157-1170. Open Access:  |
| 83. | Nucleophilic Reactivities of Schiff Base Derivatives of Amino Acids D. S. Timofeeva, A. R. Ofial, H. Mayr, Tetrahedron 2019, 75, 459-463. Access:  |
| 82.* | Synthesis, Structure and Properties of Amino-Substituted Benzhydrylium Ions - a Link Between Ordinary Carbocations and Neutral Electrophiles R. J. Mayer, N. Hampel, P. Mayer, A. R. Ofial,* H. Mayr,* Eur. J. Org. Chem. 2019, 412-421. Access:  |
| 81.* | Nucleophilicity and Electrophilicity Parameters for Predicting Absolute Rate Constants of Highly Asynchronous 1,3-Dipolar Cycloadditions of Aryldiazomethanes H. Jangra, Q. Chen, E. Fuks, I. Zenz, P. Mayer, A. R. Ofial,* H. Zipse,* H. Mayr,* J. Am. Chem. Soc. 2018, 140, 16758-16772. Access:  |
| 80.* | Intramolecular Hydrogen-Bonding Modulates the Nucleophilic Reactivity of Ammonium-Peroxycarboxylates R. J. Mayer, A. R. Ofial,* Eur. J. Org. Chem. 2018, 6010-6017. Access:  |
| 79. | Kinetics of Electrophilic Fluorinations of Enamines and Carbanions: Comparison of the Fluorinating Power of N-F Reagents D. S. Timofeeva, A. R. Ofial, H. Mayr, J. Am. Chem. Soc. 2018, 140, 11474-11486. Access:  |
| 78.* | Nucleophilic Reactivities of Bleach Reagents R. J. Mayer, A. R. Ofial,* Org. Lett. 2018, 20, 2816-2820. Access:  |
| 77.* | Kinetics and Mechanism of Oxirane Formation by Darzens Condensation of Ketones: Quantification of the Electrophilicities of Ketones Z. Li, H. Jangra, Q. Chen, P. Mayer, A. R. Ofial,* H. Zipse,* H. Mayr,* J. Am. Chem. Soc. 2018, 140, 5500-5515. Access:  |
| 76. | Quantification of the Michael-Acceptor Reactivity of α,β-Unsaturated Acyl Azolium Ions A. Levens, F. An, J. E. M. Fernando, A. R. Ofial, D. W. Lupton, H. Mayr, Top. Catal. 2018, 61, 585-590. Access:  |
| 75. | Which Factors Control the Nucleophilic Reactivities of Enamines? D. S. Timofeeva, R. J. Mayer, P. Mayer, A. R. Ofial, H. Mayr, Chem. Eur. J. 2018, 24, 5901-5910. Access:  |
| 74.* | Solvatation als Ursache für die unerwartete Nucleophilie-Reihung von Peroxid-Anionen Solvation Accounts for the Counter-Intuitive Nucleophilicity Ordering of Peroxide Anions R. J. Mayer, T. Tokuyasu, P. Mayer, J. Gomar, S. Sabelle, B. Mennucci,* H. Mayr,* A. R. Ofial,* Angew. Chem. 2017, 129, 13463-13467; Angew. Chem. Int. Ed. 2017, 56, 13279-13282. Access:  |
| 73.* | Quantification and Theoretical Analysis of the Electrophilicities of Michael Acceptors D. S. Allgäuer, H. Jangra, H. Asahara, Z. Li, Q. Chen, H. Zipse,* A. R. Ofial,* H. Mayr,* J. Am. Chem. Soc. 2017, 139, 13318-13329. Access:  |
| 72. | Nucleophilicities and Lewis Basicities of Sterically Hindered Pyridines E. Follet, H. Zipse, S. Lakhdar, A. R. Ofial, G. Berionni, Synthesis 2017, 49, 3495-3504. Access:  |
| 71. | Reactivity-Tuning in Frustrated Lewis Pairs: Nucleophilicity and Lewis Basicity of Sterically Hindered Phosphines E. Follet, P. Mayer, D. S. Stephenson, A. R. Ofial, G. Berionni, Chem. Eur. J. 2017, 23, 7422-7427. Access:  |
| 70. | Philicity, Fugality, and Equilibrium Constants: When Do Rate-Equilibrium Relationships Break Down? H. Mayr, A. R. Ofial, Pure Appl. Chem. 2017, 89, 729-744. Access:  |
| 69. | Nucleophilic Reactivities of Bis-Acceptor-Substituted Benzyl Anions Á. Puente, A. R. Ofial, H. Mayr, Eur. J. Org. Chem. 2017, 1196-1202. Access:  |
| 68. | Nucleophilicity Parameters of Stabilized Iodonium Ylides for Characterizing their Synthetic Potential S. Chelli, K. Troshin, P. Mayer, S. Lakhdar, A. R. Ofial, H. Mayr, J. Am. Chem. Soc. 2016, 138, 10304-10313. Access:  |
| 67. | Philicities, Fugalities, and Equilibrium Constants H. Mayr, A. R. Ofial, Acc. Chem. Res. 2016, 49, 952-965. Open Access:  |
| 66. | Nucleophilic Reactivities of 2-Substituted Malonates Á. Puente, S. He, F. Corral-Bautista, A. R. Ofial, H. Mayr, Eur. J. Org. Chem. 2016, 1841-1848. Access:  |
| 65.* | Sequential Oxidative α-Cyanation/Anti-Markovnikov Hydroalkoxylation of Allylamines A. Wagner, N. Hampel, H. Zipse, A. R. Ofial,* Org. Lett. 2015, 17, 4770-4773. Access:  |
| 64. | A Quantitative Approach to Polar Organic Reactivity H. Mayr, A. R. Ofial, SAR and QSAR in Environmental Research 2015, 26, 619-646. Access:  |
| 63.* | Kinetic and Theoretical Studies of Beta-Lactone Reactivity - A Quantitative Scale for Biological Application E. N. Wiedemann, F. A. Mandl, I. D. Blank, C. Ochsenfeld,* A. R. Ofial,* S. A. Sieber,* ChemPlusChem 2015, 80, 1673-1679. Access:  |
| 62.* | Benzhydrylium and Tritylium Ions: Complementary Probes for Examining Ambident Nucleophiles A. R. Ofial,* Pure Appl. Chem. 2015, 87, 341-351. Access:  |
| 61.* | Potassium Thiocyanate as Source of Cyanide for the Oxidative α-Cyanation of Tertiary Amines A. Wagner, A. R. Ofial,* J. Org. Chem. 2015, 80, 2848-2854. Access:  |
| 60. | Feineinstellung der nucleophilen Reaktivität von Bor-at-Komplexen aus Aryl- und Heteroarylboronsäureestern Fine-Tuning of the Nucleophilic Reactivities of Boron-Ate Complexes Derived From Aryl and Heteroaryl Boronic Esters G. Berionni, A. I. Leonov, P. Mayer, A. R. Ofial, H. Mayr, Angew. Chem. 2015, 127, 2820-2824; Angew. Chem. Int. Ed. 2015, 54, 2780-2783. Access:  |
| 59. | Scales of Lewis Basicities toward C-centered Lewis Acids (Carbocations) H. Mayr, J. Ammer, M. Baidya, B. Maji, T. A. Nigst, A. R. Ofial, T. Singer, J. Am. Chem. Soc. 2015, 137, 2580-2599. Open Access:  |
| 58. | Structures and Reactivities of 2-Trityl- and 2-(Triphenylsilyl)pyrrolidine-Derived Enamines: Evidence for Negative Hyperconjugation with the Trityl Group H. Erdmann, F. An, P. Mayer, A. R. Ofial, S. Lakhdar, H. Mayr, J. Am. Chem. Soc. 2014, 136, 14263-14269. Access:  |
| 57.* | Di- and Triarylmethylium Ions as Probes for the Ambident Reactivities of Carbanions Derived from 5-Benzylated Meldrum’s Acid X. Chen, Y. Tan, G. Berionni, A. R. Ofial,* H. Mayr, Chem. Eur. J. 2014, 20, 11069-11077. Access:  |
| 56. | Structures and Reactivities of Iminium Ions Derived From Substituted Cinnamaldehydes and Various Chiral Imidazolidin-4-ones F. An, S. Paul, J. Ammer, A. R. Ofial, P. Mayer, S. Lakhdar, H. Mayr, Asian J. Org. Chem. 2014, 3, 550-555. Access:  |
| 55.* | Iron-Catalyzed Generation of α-Amino Nitriles from Tertiary Amines A. Wagner, W. Han, P. Mayer, A. R. Ofial,* Adv. Synth. Catal. 2013, 355, 3058-3070. Access:  |
| 54. | Nucleophilic Reactivities and Lewis Basicities of 2-Imidazolines and Related N-Heterocyclic Compounds B. Maji, M. Baidya, J. Ammer, S. Kobayashi, P. Mayer, A. R. Ofial, H. Mayr, Eur. J. Org. Chem. 2013, 3369-3377. Access:  |
| 53.* | Towards a Comprehensive Hydride Donor Ability Scale M. Horn, L. H. Schappele, G. Lang-Wittkowski, H. Mayr, A. R. Ofial,* Chem. Eur. J. 2013, 19, 249-263. Access:  |
| 52. | A quantitative approach to nucleophilic organocatalysis H. Mayr, S. Lakhdar, B. Maji, A. R. Ofial, Beilstein J. Org. Chem. 2012, 8, 1458-1478. Access:  |
| 51.* | No Detours: Palladium-Catalyzed Oxidative C-H/C-H Cross-Couplings of Heteroarenes W. Han, A. R. Ofial,* Synlett 2011, 1951-1955. Access:  |
| 50.* | Palladium-Catalyzed Direct Arylations of Azoles with Aryl Silicon and Tin Reagents W. Han, P. Mayer, A. R. Ofial,* Chem. Eur. J. 2011, 17, 6904-6908. Access:  |
| 49.* | Palladium-katalysierte dehydrierende Kreuzkupplung von Benzazolen mit Azolen Palladium-Catalyzed Dehydrogenative Cross Couplings of Benzazoles with Azoles W. Han, P. Mayer, A. R. Ofial,* Angew. Chem. 2011, 123, 2226-2230; Angew. Chem. Int. Ed. 2011, 50, 2178-2182. Access:  |
| 48. | Abschied vom HSAB-Modell ambidenter Reaktivität Farewell to the HSAB Treatment of Ambident Reactivity H. Mayr, M. Breugst, A. R. Ofial, Angew. Chem. 2011, 123, 6598-6634; Angew. Chem. Int. Ed. 2011, 50, 6470-6505. Angew. Chem. 2011, 123, 6598-6634; Access:  |
| 47. | The Origin of Ultrafast Proton Transfer: Multidimensional Wave Packet Motion vs. Tunneling C. Schriever, S. Lochbrunner, A. R. Ofial, E. Riedle, Chem. Phys. Lett. 2011, 503, 61-65. Access:  |
| 46.* | Electrophilic Reactivities of 1,2-Diaza-1,3-dienes T. Kanzian, S. Nicolini, L. De Crescentini, O. A. Attanasi,* A. R. Ofial,* H. Mayr, Chem. Eur. J. 2010, 16, 12008-12016. Access:  |
| 45.* | Iron-Catalyzed Oxidative Mono- and Bis-Phosphonation of N,N-Dialkylanilines W. Han, P. Mayer, A. R. Ofial,* Adv. Synth. Catal. 2010, 352, 1667-1676. Access:  |
| 44. | Reactivity Parameters for Rationalizing Iminium-Catalyzed Reactions S. Lakhdar, A. R. Ofial, H. Mayr, J. Phys. Org. Chem. 2010, 23, 886-892. Access:  |
| 43. | trans-1-Phenylpyrrolidine-2,5-dicarbonitrile W. Han, A. R. Ofial, P. Mayer, Acta Crystallogr., Sect. E: Struct. Rep. Online 2010, 66, o397. Access:  |
| 42.* | Iron catalyzed dehydrogenative phosphonation of N,N-dimethylanilines W. Han, A. R. Ofial,* Chem. Commun. 2009, 6023-6025. Access:  |
| 41.* | Iron catalyzed oxidative cyanation of tertiary amines W. Han, A. R. Ofial,* Chem. Commun. 2009, 5024-5026. Access:  |
| 40. | 4-[4-(Dimethylamino)benzylidene]-2,6-dimethylcyclohexa-2,5-dienone N. Hampel, D. Richter, A. R. Ofial, H. Mayr, P. Mayer, Acta Crystallogr., Sect. E: Struct. Rep. Online 2009, 65, o2102. Access:  |
| 39. | Can One Predict Changes from SN1 to SN2 Mechanisms? T. B. Phan, C. Nolte, S. Kobayashi, A. R. Ofial, H. Mayr, J. Am. Chem. Soc. 2009, 131, 11392-11401. Access:  |
| 38. | Synthesis and Characterization of Novel Quinone Methides: Reference Electrophiles for the Construction of Nucleophilicity Scales D. Richter, N. Hampel, T. Singer, A. R. Ofial, H. Mayr, Eur. J. Org. Chem. 2009, 3203-3211. Access:  |
| 37. | How To Predict Changes in Solvolysis Mechanisms H. Mayr, A. R. Ofial, Pure Appl. Chem. 2009, 81, 667-683. Access:  |
| 36. | Und es geht doch: Nucleophilieskalen für die Syntheseplanung H. Mayr, A. R. Ofial, Nachr. Chem. 2008, 56, 871-877. Access:  |
| 35. | Nucleophilic Reactivities of Pyrroles T. A. Nigst, M. Westermaier, A. R. Ofial, H. Mayr, Eur. J. Org. Chem. 2008, 2369-2374. Access:  |
| 34. | Do General Nucleophilicity Scales Exist? H. Mayr, A. R. Ofial, J. Phys. Org. Chem. 2008, 21, 584-595. Access:  |
| 33. | Inverse Solvent Effects in Carbocation Carbanion Combination Reactions: The Unique Behavior of Trifluoromethylsulfonyl Stabilized Carbanions S. T. A. Berger, A. R. Ofial, H. Mayr, J. Am. Chem. Soc. 2007, 129, 9753-9761. Access:  |
| 32. | Nucleophilic Reactivities of Indoles S. Lakhdar, M. Westermaier, F. Terrier, R. Goumont, T. Boubaker, A. R. Ofial, H. Mayr, J. Org. Chem. 2006, 71, 9088-9095. Access:  |
| 31. | Das Reaktivitäts-Selektivitäts-Prinzip: ein unzerstörbarer Mythos der organischen Chemie The Reactivity-Selectivity Principle: An Imperishable Myth in Organic Chemistry H. Mayr, A. R. Ofial, Angew. Chem. 2006, 118, 1876-1886; Angew. Chem. Int. Ed. 2006, 45, 1844-1854. Access:  |
| 30. | How Fast Do R-X Bonds Ionize? - A Semiquantitative Approach B. Denegri, A. R. Ofial, S. Juric, A. Streiter, O. Kronja, H. Mayr, Chem. Eur. J. 2006, 12, 1657-1666. Access:  |
| 29. | Kinetics of the Solvolyses of Benzhydryl Derivatives: Basis for the Construction of a Comprehensive Nucleofugality Scale B. Denegri, A. Streiter, S. Juric, A. R. Ofial, O. Kronja, H. Mayr, Chem. Eur. J. 2006, 12, 1648-1656; 5415. Access:  |
| 28. | Quantification of the β-Stabilizing Effect of the Dicarbonyl(η5-cyclopentadienyl)iron Group F. Dulich, K.-H. Müller, A. R. Ofial, H. Mayr, Helv. Chim. Acta 2005, 88, 1754-1768. Access:  |
| 27. | Kinetics of Electrophile-Nucleophile Combinations: A General Approach to Polar Organic Reactivity H. Mayr, A. R. Ofial, Pure Appl. Chem. 2005, 77, 1807-1821. Access:  |
| 26. | The Propagation Rate of the Cationic Polymerization of 2,4,6-Trimethylstyrene: A Linear Free Energy Approach H. Mayr, A. R. Ofial, H. Schimmel, Macromolecules 2005,38, 33-40. Access:  |
| 25. | Determination of Rate Constants in the Carbocationic Polymerization of Styrene: Effect of Temperature, Solvent Polarity, and Lewis Acid P. De, R. Faust, H. Schimmel, A. R. Ofial, H. Mayr, Macromolecules 2004, 37, 4422-4433. Access:  |
| 24. | Stereoselective synthesis of cis-fused hexahydro-isoindolones S. Rehn, A. R. Ofial, K. Polborn, H. Mayr, ARKIVOC 2004 (iii), 120-131. Access:  |
| 23. | Reactivities of Carbocations and Carbanions A. R. Ofial, H. Mayr, Macromol. Symp. 2004, 215, 353-367. Access:  |
| 22. | Electrophilicity Scales for Carbocations H. Mayr, A. R. Ofial in Carbocation Chemistry (G. A. Olah, G. K. S. Prakash, Eds.); Wiley: Hoboken, NJ, 2004; Chapter 13, pp 331-358. Access:  |
| 21. | Synthesis of Allylamines from Alkynes and Iminium Ions S. Rehn, A. R. Ofial, H. Mayr, Synthesis 2003, 1790-1796. Access:  |
| 20. | Role of Electron Transfer Processes in Reactions of Diarylcarbenium Ions and Related Quinone Methides with Nucleophiles A. R. Ofial, K. Ohkubo, S. Fukuzumi, R. Lucius, H. Mayr, J. Am. Chem. Soc. 2003, 125, 10906-10912. Access:  |
| 19. | Structure-Nucleophilicity Relationships for Enamines B. Kempf, N. Hampel, A. R. Ofial, H. Mayr, Chem. Eur. J. 2003, 9, 2209-2218. Access:  |
| 18. | 5-Methoxyfuroxano[3,4-d]pyrimidine: a highly reactive neutral electrophile G. Ya. Remennikov, B. Kempf, A. R. Ofial, K. Polborn, H. Mayr, J. Phys. Org. Chem. 2003, 16, 431-437. Access:  |
| 17. | π-Nucleophilicity in Carbon-Carbon Bond Forming Reactions H. Mayr, B. Kempf, A. R. Ofial, Acc. Chem. Res. 2003, 36, 66-77. Access:  |
| 16. | Determination of the electrophilic reactivities of 1,1,3-triarylallyl cations H. Mayr, C. Fichtner, A. R. Ofial, J. Chem. Soc., Perkin Trans. 2 2002, 1435-1440. Access:  |
| 15. | Initiation and Propagation Rate Constants for the Cationic Polymerization of N-Vinylcarbazole H. Schimmel, A. R. Ofial, H. Mayr, Macromolecules 2002, 35, 5454-5458. Access:  |
| 14. | Reactions of Carbocations with Unsaturated Hydrocarbons: Electrophilic Alkylation or Hydride Abstractions? H. Mayr, G. Lang, A. R. Ofial, J. Am. Chem. Soc. 2002, 124, 4076-4083. Access:  |
| 13. | Wegweiser im Dschungel Organischer Reaktivität H. Mayr, A. R. Ofial, Einsichten - Forschung an der LMU München 2001, 20, 30-33. Access:  |
| 12. | Reference Scales for the Characterization of Cationic Electrophiles and Neutral Nucleophiles H. Mayr, T. Bug, M. F. Gotta, N. Hering, B. Irrgang, B. Janker, B. Kempf, R. Loos, A. R. Ofial, G. Remennikov, H. Schimmel, J. Am. Chem. Soc. 2001, 123, 9500-9512. Access:  |
| 11. | Reactions of Carbon Electrophiles with Cobalt-Coordinated Enynes: Scope and Limitations H. Mayr, O. Kuhn, C. Schlierf, A. R. Ofial, Tetrahedron 2000, 56, 4219-4229. Access:  |
| 10. | [2++4] Cycloadditions of Iminium Ions - Concerted or Stepwise Mechanism of Aza Diels-Alder Reactions? H. Mayr, A. R. Ofial, J. Sauer, B. Schmied, Eur. J. Org. Chem. 2000, 2013-2020. Access:  |
| 9. | Comparison of the Electrophilicities of the Free and the (Tricarbonyl)iron-Coordinated Tropylium Ion H. Mayr, K.-H. Müller, A. R. Ofial, M. Bühl, J. Am. Chem. Soc. 1999, 121, 2418-2424. Access:  |
| 8. | Reactivities and Selectivities of Free and Metal-Coordinated Carbocations H. Mayr, M. Patz, M. F. Gotta, A. R. Ofial, Pure Appl. Chem. 1998, 70, 1993-2000. Access:  |
| 7. | NMR Spectroscopic Evidence for the Structure of Iminium Ion Pairs H. Mayr, A. R. Ofial, E.-U. Würthwein, N. C. Aust, J. Am. Chem. Soc. 1997, 119, 12727-12733. Access:  |
| 6. | Electrophilicities of Iminium Ions H. Mayr, A. R. Ofial, Tetrahedron Lett.1997, 38, 3503-3506. Access:  |
| 5. | A Novel Pentaannulation Reaction of Iminium Ions A. R. Ofial, H. Mayr, Liebigs Ann. 1997, 333-335. Access:  |
| 4. | X-ray and 35Cl NQR Studies on the Trichloroacetyl Group in Covalent and Ionic Compounds of L-Valine and DL-Valine A. R. Ofial, S.-q. Dou, V. G. Krishnan, H. Paulus, H. Fuess, Al. Weiss, Z. Naturforsch. A 1997, 52, 249-258. Access:  |
| 3. | En-Reaktionen von Alkinen zur stereoselektiven Synthese von Allylaminen Ene Reactions of Alkynes for the Stereoselective Synthesis of Allylamines A. R. Ofial, H. Mayr, Angew. Chem. 1997, 109, 145-147; Angew. Chem. Int. Ed. Engl. 1997, 36, 143-145. Access:  |
| 2. | Reactions of Allylsilanes with Iminium Salts: Ene Reactions with Inverse Electron Demand A. R. Ofial, H. Mayr, J. Org. Chem. 1996, 61, 5823-5830. Access:  |
| 1. | N-Trichloro- and dichloroacetyl amino acids and compounds of amino acids with halogeno acetic acids: 35Cl nuclear quadrupole resonance spectroscopy; crystal structure of N-trichloroacetyl-glycine, -DL-alanine, and -L-alanine S.-q. Dou, A. Kehrer, A. R. Ofial, Al. Weiss, J. Mol. Struct. 1995, 345, 11-29. Access:  |
Publications | Database | Reactivity Scales Poster
Database
Mayr's Database of Reactivity Parameters (regularly updated)
Publications | Database | Reactivity Scales Poster
Reactivity Scales Posters
Different poster versions of the reactivity scales are available:
(a) Mayr's Reactivity Scales Poster 2023 [pdf] (version: 08/2023)
(b) The 'old style' poster [pdf] (version: 03/2016)
(c) Reactivity scales for electrophiles and nucleophiles used in organocatalysis and organometallic chemistry [pdf] (version: 03/2016)